Risk of too much or too little insulin causing low or high blood sugar, loss of consciousness, death.
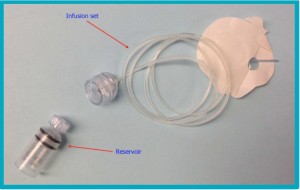 People with diabetes use insulin to regulate their blood sugar levels. Insulin can be given by injection, but many have found an easier, more constant delivery of insulin (and no need to continue to draw blood to test levels) by using an insulin pump that attaches to their clothing at waist level.
People with diabetes use insulin to regulate their blood sugar levels. Insulin can be given by injection, but many have found an easier, more constant delivery of insulin (and no need to continue to draw blood to test levels) by using an insulin pump that attaches to their clothing at waist level.
Although this is a popular option, there are risks. Defects in the tubing of the Medtronic insulin pump (infusion sets) could cause over or under delivery of insulin. The dangerous result could be hypo- or hyper- glycemia (low or high blood sugar), loss of consciousness, and possibly death.
If you or someone you know has experienced these injuries or side effects, call 800-883-2299 or fill out the inquiry form on this page.
What is the defect?
If insulin or other fluids come in contact with the inside of the tubing connector it can temporarily block the vents that allow the pump to properly prime. The FDA recommends that if you notice anything unusual during the infusion set prime process such as the insulin continuing to drip from the tip of the infusion set cannula when priming has been completed, this may indicate that the connector vents are not working properly. See below for the model numbers affected.
When was the defect found?
The July 2013 FDA Class 1 recall states “if patients notice anything unusual during the infusion set prime process such as the insulin continuing to drip from the tip of the infusion set cannula when priming has been completed, this may indicate that the connector vents are not working properly. If this occurs, do not insert the infusion set.” Medtronic, the manufacturer of the MiniMed Paradigm Insulin Infusion Pump issued an urgent safety notification, also in July 2013. There were reports of injury and death prior to the recall and safety notification.
What do I do if I believe my pump is malfunctioning?
Get immediate medical help to make sure your blood sugar is stable. Keep the infusion set that caused the proplem, and if you get a new pump, keep the old pump- do not give it to a health care professional or the manufacturer. It may need to be evaluated for the defect.
The defective medical device attorneys of Sheller, P.C. are available to you for a no cost, no obligation consultation. Fill out the inquiry form on this page or call 800-883-2299.
What models are affected?
Medtronic MiniMed Paradigm Insulin Infusion Sets
Models: MMT-317, MMT-318, MMT-324, MMT-325, MMT-312S, MMT-312L, MMT-386, MMT-387, MMT-394, MMT-396, MMT-397, MMT-398, MMT-399, MMT-377, MMT-378, MMT-381, MMT-382, MMT-383, MMT-384, MMT-368, MMT-862, MMT-864, MMT-866, MMT-874, MMT-876, MMT-884, MMT-886, MMT-921, MMT-923, MMT-925, MMT-941, MMT-943, MMT-945, MMT-961, MMT-963, MMT-965, & MMT-975 Paradigm Infusion sets.
These affected products were manufactured from October, 2001 through June, 2013 and distributed from December, 2001 through June, 2013.
Link
FDA Class 1 Recall: Medtronic MiniMed Paradigm Insulin Infusion Sets: Class 1 Recall – Potential for Over or Under Delivery of Insulin
