Invokana: FDA Warnings on SGLT2 Diabetes Drugs
Sheller, P.C. attorneys are investigating the newer class of diabetes drugs following FDA warnings. ABOUT THE DRUGS Invokana (Invokamet) is a relatively new SGLT2 inhibitor diabetes drug that is intended to be used in combination with diet and exercise to improve glycemic control and lower blood sugar in adults with type 2 diabetes mellitus. When […]
$58.9 MILLION SETTLEMENT
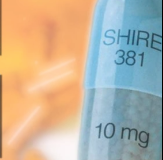
The Sheller whistleblower team announced a $58.9 milllion settlement with Shire Pharmaceuticals over off-label marketing of ADHD drugs Adderall and Vyvanse in September, 2014. Court documents detailing the civil allegations and settlement were signed by Shire and unsealed by the Office of the United States Attorney for the Eastern District of Pennsylvania. A Shire executive, […]
Sheller Spars With FDA

Lawyer Spars With FDA Over J&J Risperdal Court Documents by Ed Silverman, Pharmalot In an unusual tactic, an attorney is prodding the FDA to seek documents that purportedly detail side effects caused by the Risperdal antipsychotic, but cannot be released publicly due to a court order. The documents were sealed by a Philadelphia judge as […]
In The New York Times: “Side Effects May Include Lawsuits”

One of the most-read articles in The New York Times regarding pharmaceutical drug product liability and whistleblower lawsuits quotes Stephen Sheller. Side Effects May Include Lawsuits BY DUFF WILSON NEW YORK – FOR decades, antipsychotic drugs were a niche product. Today, they’re the top-selling class of pharmaceuticals in America, generating annual revenue of about $14.6 billion […]





