PA Supreme Court Will Hear Appeal of Risperdal Time-Bar
The PA Supreme Court has agreed to review a Superior Court decision which would dismiss thousands of Risperdal cases due to statute of limitations. The two cases on appeal, Saksek v. Janssen and Winter v. Janssen, were dismissed for failing to bring a claim with in the applicable two year statue of limitations. The Supreme Court’s decision would […]
$4.7B Talc-Ovarian Cancer Verdict Against J&J

Juries across the country have been handing down “eye catching” verdicts for plaintiffs with ovarian cancer and their families against Johnson & Johnson. The most recent included a nearly $4.7 billion verdict for 22 plaintiffs in Missouri. Plaintiff’s claim J&J knowingly, for 49 years, sold talcum baby powder tainted with asbestos which resulted in the […]
Judge Urges Appeals Court to Uphold $70M Risperdal Verdict
Philadelphia Court of Common Pleas Judge Paula Patrick urges the Pennsylvania Superior Court to uphold the $70 Million jury verdict in A.Y. v. Janssen Pharmaceuticals. AY is one of many boys who developed gynecomastia after taking Janssen’s anti-psychotic medication Risperdal. This is the 5th trial for Risperdal, with over 6,000 awaiting trial in a consolidated […]
MI Law May Prevent Recovery of Millions Spent Fighting Opioid Crisis
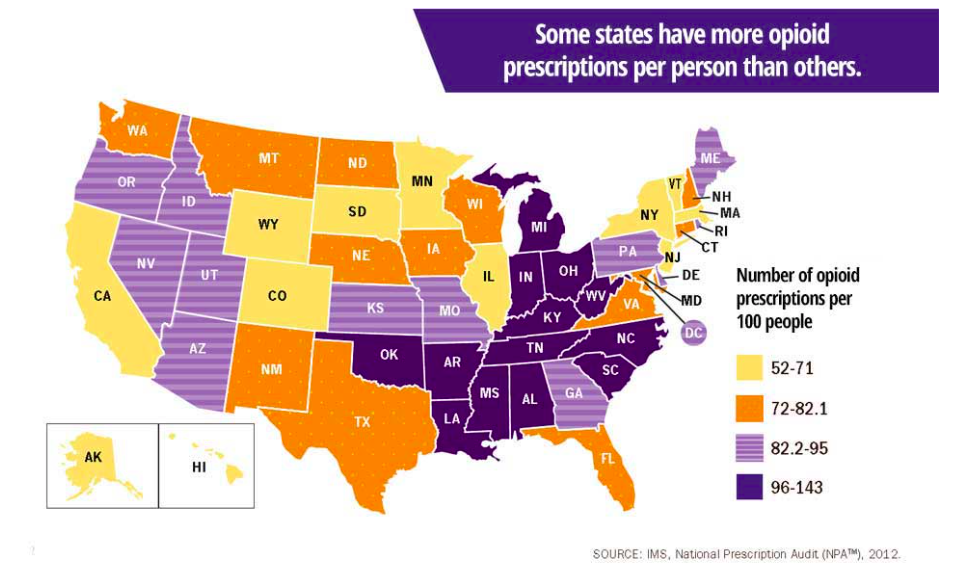
As the nation’s opioid crisis continues 162 of the 600 people recently charged with healthcare fraud were healthcare professionals who contributed to the nation’s fastest and most deadly epidemic. Conducted nationwide, this was the largest health care fraud investigation. Last year, more than 400 people were charged, 115 of them were doctors, nurses and other healthcare professionals […]
Privacy policy updated to comply with GDPR

Sheller.com’s privacy policy is hereby updated to reflect the new European Union General Data Protection Regulations (“GDPR”). This policy describes how Sheller, P.C. collects and uses the identifiable and non-identifiable information collected on sheller.com. Information you provide “offline” is not generally subject to this policy, contact us at info@sheller.com. This Privacy Policy does not describe Sheller P.C.’s […]
Sheller Attorneys 2018 Super Lawyers

Super Lawyers has again recognized the attorneys of Sheller P.C. in 2018. Founder Stephen A. Sheller has been selected by the organization for the fifteenth consecutive year. Attorney Jamie L. Sheller have been awarded the honor multiple years, including 2018. Super Lawyers is a rating service of outstanding lawyers from more than 70 practice areas across […]
Sheller Panelist on Penn Law’s Legal Response to Opioid Conference

Sheller PC’s founder, Stephen Sheller, joined other litigators, judges and state prosecutors at a conference to discuss the legal response to the opioid crisis. The forum was hosted by University of Pennsylvania Law School’s Center for Ethics and the Rule of Law (CERL). The panel in which Sheller participated, Corporate Responsibility and Civil Law Solutions, […]
Sheller honored with Drexel Degree, Doctor of Humane Letters

Stephen A. Sheller, who has served on the board of trustees of Drexel University since 2003 and on Drexel’s Thomas R. Kline School of Law advisory board, will receive an honorary degree at the Drexel University commencement ceremony on June 13, 2017 at Citizen’s Bank Ballpark in Philadelphia. For his commitment to championing the disenfranchised, […]
Sheller Joins Phila. in Suit Against Opioid Manufacturers

Sheller PC is one of five private law firms assisting the city of Philadelphia’s Law Department in its suit against pharmaceutical companies attempting to hold them accountable for the current opioid crisis. Five pharmaceutical companies are named in the suit: Allergan, Janssen (a subsidiary of Johnson & Johnson and manufacturer of Risperdal), Endo, Cephalon, and […]
Superior Court Allows Punitive Damages in Risperdal Mass Torts

In a significant ruling by the Pennsylvania Superior Court, Janssen, a subsidiary of Johnson & Johnson (J&J), will now face punitive damages in Risperdal mass tort suits. A previous Philadelphia trial court ruling prohibited punitive damages. Boys and young men who developed gynecomastia, female-like breasts, from taking the antipsychotic drug and whose cases had already […]





