
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
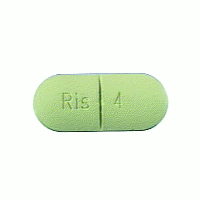
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
Diabetes drug being investigated for potentially fatal bladder cancer risk
ACTOS is the brand name of a drug developed by Takeda Pharmaceuticals in conjunction with Eli Lilly and Company. The drug is designed to help control blood sugar levels in patients with Type 2 Diabetes. The generic name of the drug is pioglitazone.
Sheller attorneys are investigating the serious and potentially fatal risks of ACTOS. Contact the firm at 800-883-2299.
NEWS UPDATE: Actos manufacturer Takeda is said to have agreed to a $2.2 billion settlement for patients harmed by the drug
If you take or have taken ACTOS and have experienced these symptoms, consult your health care provider immediately:
If you have a diagnosis of bladder cancer and have been taking ACTOS for more than one year, contact us now by calling 800-883-2299, filling out the form on this page or e-mailing us for a no obligation consultation about your legal rights.
Links
FDA Drug Safety Review Update, June 2011
Takeda Offers $2.2 Billion to Settle Actos Drug Cases, Bloomberg News, March 31, 2015