
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
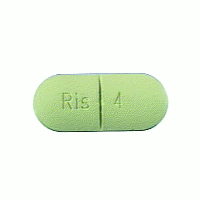
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
Paxil (paroxetine hydrochloride), also known as Seroxat in Europe, is manufactured by GlaxoSmithKline. It is a prescription drug for the treatment of depression, panic disorder, social phobia, and obsessive-compulsive disorder, among other ailments. Paxil is part of a class of drugs called Selective Serotonin Reuptake Inhibitors (SSRI). The FDA approved this drug in 1992.
Since 2003, the FDA has notified healthcare professionals of reports of the occurrence of suicidality (both suicidal ideation and suicide attempts) in clinical trials for various antidepressant drugs in pediatric patients with major depressive disorder (MDD) which included Paxil. Scientists expressed concern about birth defects over ten years but Glaxo did not disclose the information as required to the FDA.
Approved by the FDA in 1992, Glaxo scientists raised concerns about birth defects as early as 1997 but Glaxo executives withheld the information from the FDA despite requirements to disclose the evidence. Glaxo reported nearly $1 billion in Paxil sales in 2008 alone.
Taking Paxil while undergoing chemotherapy with the drug tamoxifen:
The British Medical Journal in February 2010 published a study indicating that women who took Paxil during tamoxifen chemotherapy may have up to an 90% increased risk of death. The researchers said there appeared to be no such link with other antidepressants but there might not have been enough women studied to indicate the risk/benefit of the other SSRI drugs. Warnings were issued to doctors to advise patients on the risks, and if indicated slowly reduce the dosage of Paxil to avoid serious withdrawal side effects.
Paxil and other SSRI’s have been linked with serious, sometimes fatal, reactions. These reactions include:
While many healthcare providers advise consumers to take a drug for the long term any “lifelong” prescription, consumers are advised to research safety and talk with their healthcare professional about the risk-benefit of the drug treatment as well as the challenges and dangers of changing dosages of this or any drug. Ask your healthcare professional about other therapies for depression. There are other options that do not create problems. Recent studies in other medical journals report that for some patients antidepressants are no more successful than a placebo or non-drug therapy for depression.
Sheller attorney prevails in landmark, first Paxil birth defects case: recent Paxil-related trials have drawn international attention. Attorney Jamie L. Sheller, local counsel in the Kilker matter heard in the Philadelphia court system, represented a mother who says Paxil manufacturer GlaxoSmithKline continued to promote the drug while withholding evidence that it caused significant birth defects. Continuing to take Paxil while pregnant after being assured it was safe, the client’s son was hospitalized and required heart surgery following birth and will require more surgery. Plaintiffs prevailed, securing a $2.5 million trial verdict in the Philadelphia Court of Common Pleas litigating. It was the first of 600 cases regarding the antidepressant Paxil and birth defects.
Many plaintiffs contend that GlaxoSmithKline deliberately failed to warn both patients and physicians of the potential birth defects, chemically addictive nature of the drug and the withdrawal reactions that can occur with Paxil®, even if the patient is prescribed a new antidepressant. It’s believed the “half-life,” or the amount of time the drug stays in the body, contributes to prolonging the severe withdrawal symptoms.
The American Law Journal: Jamie Sheller discusses $2.5 million verdict in Paxil birth defects case
WHYY National Public Radio: Jamie Sheller interviewed on Paxil birth defects case
Philadelphia Inquirer: Jamie Sheller interviewed on Paxil birth defects case
CBS News: Paxil blocks cancer drug’s effects
WebMD: Paxil and Tamoxifen may be a risky combo
IF YOU TAKEN PAXIL OR AN SSRI DRUG WHILE PREGNANT OR UNDERGOING CHEMOTHERAPY AND HAVE EXPERIENCES HEART PROBLEMS, STROKE, SERIOUS PSYCHIATRIC SIDE EFFECTS, IF YOUR LOVED ONE OR SOMEONE YOU KNOW WAS TAKING PAXIL® WHILE UNDERGOING CHEMOTHERAPY WITH THE DRUG TAMOXIFEN AND SUBSEQUENTLY DIED OF BREAST CANCER, THERE MAY BE A CONNECTION. CONTACT SHELLER, P.C. ATTORNEYS AT 800-883-2299 OR A CONFIDENTIAL FORM BELOW, TO PROTECT YOUR HEALTH AND POSSIBLY RECEIVE A COMPENSATION.