
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
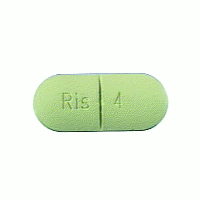
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
Remicade (infliximab) is manufactured by Centocor Inc. (a subsidiary of Janssen/Johnson & Johnson); it is an immune-suppressing drug that is used to treat rheumatoid arthritis and Crohn’s disease in patients. It works by blocking your immune system’s overproduction of a protein (TNF-alpha), which is an underlying cause of these diseases. Remicade targets and neutralizes TNF-alpha to relieve those painful symptoms. But by purposely impeding the TNF, it is believed that the drug reduces the body’s immunity against other diseases, allowing the side effects to arise that can cause life-threatening and possibly fatal outcomes. It is often associated with Enbrel.
If you or anyone you know has taken Remicade or a generic version and experienced a serious infection, contact us immediately using the inquiry form on this page or call 800-883-2299 to protect your health now and in the future.
The FDA required a drug labeling change stating that Remicade and similar “inhibitor” drugs may put patients (pediatric up to elderly populations) at increased risk of bacterial infections including Legionella and Listeria. The infections occured either within a month or even as late as six years after starting the drugs. These are potentially life-threatening infections, and may require patients be put on ventilators, in intensive care, and more.
Remicade was approved for the treatment of Crohn’s disease in 1998 and for rheumatoid arthritis in 2000. Since then Centocor says it has learned of several reports of blood and nervous system disorders, including some fatal cases, in people taking the drug.
In a warning letter sent to health care providers in August 2004, the company said some Remicade users suffered from a loss of infection-fighting white blood cells, oxygen-carrying red blood cells, and blood-clotting platelets that left them vulnerable to infection or abnormal bleeding, and some of them died.
The company also warned of rare cases of central nervous system disorders, such as inflammation of the blood vessels that have been reported in Remicade users. In response, Centocor and the FDA have revised the warnings and adverse reactions sections of the labeling for Remicade to include information on these possible risks.
Important safety information has been added by Centocor and the FDA to the label of the rheumatoid arthritis drug. Based on worldwide post-marketing experience the following blood (or hematologic) events, sometimes fatal, were reported in patients receiving Remicade. It should be noted that Enbrel (from Amgen, Inc.) and Humira (from Abbott Laboratories, Inc.) carry similar warnings on their labels regarding blood disorders. Remicade, Enbrel, and Humira are anti-TNF drugs and work by blocking tumor necrosis factor (TNF), a protein involved in causing inflammation.
Officials say people using Remicade should seek immediate medical attention if they develop symptoms of blood disorders or infection while using the drug, such as:
IF YOU OR SOMEONE YOU LOVE EXPERIENCED AN ADVERSE REACTION AS A RESULT OF TAKING REMICADE, CONTACT OUR OFFICE IMMEDIATELY AT 800-883-2299 OR FILL OUT A CONFIDENTIAL FORM BELOW.