
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
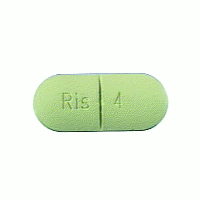
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
Topamax (topiramate) was originally approved by the FDA in 1996 as an epilepsy and anti-seizure drug and in preventing migraines in adults. In 2012, the drug was approved and as a weight loss and eating disorder treatment. Off-label, the drug has been prescribed for psychiatric conditions including bipolar disorder, mood swings, obsessive compulsive and borderline personality disorders, eating disorders including binge eating, and alcohol, drug, tobacco and other addictions.
Attorney Jamie Sheller was on the trial team that successfully litigated another birth defects case involving an antidepressant. She and the firm have a special affinity for mothers and injury to their babies and pledge their full attention to these cases. Fill out the inquiry form on this page or call 800-883-2299; there may be a time limit to file a claim.
November 18, 2013: A jury in Philadelphia awarded an $11 million verdict in a lawsuit claiming the anti-seizure drug Topamax was responsible for a cleft lip birth defect. This verdict follows an earlier $4 million awarded to a Virginia woman in late October whose son was also born with a cleft lip. See links below for news stories.
Class action and mass tort lawsuits have kept many defective products off the market and penalized manufacturers who intentionally sold them. Class action lawsuits have rid our schools of dangerous asbestos and eliminated harmful contraceptive devices; they have made cars safer, products more reliable and given consumers a power to effect the market that they would not have otherwise. Mass torts have played a large and very successful role in the relief and compensation for people injured by dangerous pharmaceutical drugs and defective medical devices. Sheller, P.C. attorneys have a large dangerous drug and defective medical device practice where lawsuits often become mass torts. Call 800-883-2299 for more information on whether your situation qualifies for these type of legal actions.
In response to the incident reports, the FDA required Johnson & Johnson to increase the warning from a category “C” to “D,” the most serious warning for the potential of birth defects. FDA warning states that TOPAMAX can cause fetal harm when administered to a pregnant woman. Data from pregnancy registries indicate that infants exposed to topiramate in utero have an increased risk for cleft lip and/or cleft palate (oral clefts).
Manufactured by the Johnson & Johnson subsidiary Janssen Pharmaceuticals, the drug first came under scrutiny as an alleged cause of birth defects including cleft lip and cleft palate, deformities of the mouth that can affect breathing, eating, talking and other functions. The baby’s lip and/or palate in the roof of their mouth do not join before birth, leaving an open groove or split lip and skin.
In 2011, WebMD noted that drug registry data indicated the oral birth defects were sixteen times higher in mothers who took Topamax or the generic topiramate. Of mothers who took the drug, 1.4% of babies developed the birth defects, compared to .35-.55% of infants whose mothers took other epilepsy/anti-seizure drugs.
Patients who are currently taking Topamax or the generic topiramate should not stop without first consulting with a healthcare professional as there may be significant risks to suddenly stop taking the drug.
IF YOUR BABY OR ONE THAT YOU KNOW WAS BORN WITH BIRTH DEFECTS AFTER THE MOTHER TOOK TOPAMAX, CONTACT THE DEFECTIVE DRUG ATTORNEYS AT SHELLER, P.C. AT 800-883-2299 OR FILL OUT THE CONFIDENTIAL FORM BELOW TO LEARN YOUR LEGAL RIGHTS AND WHETHER YOU ARE ELIGIBLE FOR COMPENSATION.
“J&J’s Janssen Loses $11 Million Jury Verdict Over Topamax” Bloomberg News, November 18, 2013
“Jury Awards $4 Million on Claim Janssen Drug Caused Birth Defect” Bloomberg News, October 30, 2013
“FDA: Risk of Oral Birth Defects in Children Born to Mothers Taking Topiramate” FDA News and Events, March 4, 2011
“New Birth Defect Warning for Topamax” WebMD, March 4, 2011