
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
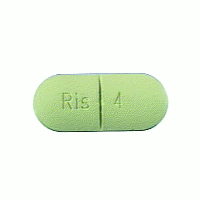
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
The attorneys of Sheller, P.C. are investigating the serious risks of Xarelto and Pradaxa. The firm offers no cost, no obligation consultations to individuals who have experienced the serious side effect of excessive, uncontrollable bleeding. If you or someone you know has experienced this dangerous side effect, call 800-883-2299 or fill out the inquiry form at the bottom of this page.
Xarelto and Pradaxa are prescription drugs used as a blood thinner to reduce the risk of stroke and blood clots.
Prescription blood thinners have been in use since the 1950’s, with the most recognized brand name being Coumadin. Indeed, anticoagulants can be a useful therapy for patients, but they come with a risk of bleeding, and that’s where Xarelto is different. However, newer blood thinning drugs Xarelto and Pradaxa, both come along with different and sometimes life-threatening risks.
The problem lies in the one key difference with new group of blood thinners says an article on the pharmaceutical industry website Fierce Pharma: “Warfarin’s effects can be quickly reversed with an antidote (to stop the bleeding). That’s not true of the new drugs, Xarelto and Pradaxa.”
Thus, these drugs thin the blood as intended but may lead to uncontrollable bleeding with little to no “fix” to stop the bleeding at home, in a doctor’s office, or even at a hospital. In other words, if a patient begins bleeding on Xarelto, there is no antidote.
Moreover, Xarelto has not been shown to work substantially better than warfarin nor has it been shown to be safer. Nonetheless, in part due to heavy marketing by the manufacturers, Xarelto is now the most prescribed blood thinner after warfarin.
As of early 2015, several hundred lawsuits had been filed, with the bulk of the cases filed in the federal multidistrict litigation in the Eastern District of Louisiana or in the Complex Litigation Center in the Philadelphia Court of Common Pleas. To learn more about Xarelto lawsuits and and why you should file a lawsuit, visit http://BloodThinnerHelp.com
Bayer, J&J face calls to consolidate Xarelto blood Thinner lawsuits, December 4, 2014, Fierce Pharma
Bayer’s Xarelto faces stepped-up reports of side effects, deaths, September 9, 2013, Fierce Pharma
JAMA says FDA jumped the gun on Gilenya, Pradaxa, September 5, 2012, Fierce Pharma
FDA delivers Xarelto bad news to J&J, Bayer, June 22, 2012, Fierce Pharma
Top heart doctors fret over new blood thinners, June 14, 2012, Reuters
Heart docs skeptical of new-generation clotbusters: Familiar with warfarin problems, physicians worry about Pradaxa®, Xarelto® risks, June 14, 2012, Fierce Pharma
New blood thinner Pradaxa may cause internal bleeding in the elderly, June 15, 2012, Philly.com
U.S. advisers reject J&J/Bayer’s Xarelto® for acute coronary patients, May 23, Reuters
Study: Higher heart attack risk from Pradaxa, January 11, 2012, CBS.com