
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
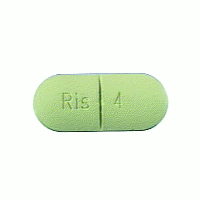
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
 Zofran (ondansetron) is a drug given to women during pregnancy to ease nausea and morning sickness. Although it has been prescribed for this purpose, it is not FDA approved for pregnant women. In fact, the drug is listed as a “Class B” substance generally meaning there has not been enough research on a larger number of women and their babies to know the extent of the risks.
Zofran (ondansetron) is a drug given to women during pregnancy to ease nausea and morning sickness. Although it has been prescribed for this purpose, it is not FDA approved for pregnant women. In fact, the drug is listed as a “Class B” substance generally meaning there has not been enough research on a larger number of women and their babies to know the extent of the risks.
Some families who have children born with birth defects have not associated use of Zofran with the birth defects. Contact our firm so that we can investigate if there is a potential link. Sheller, P.C. collaborates with doctors to help families find the truth and if eligible, compensation and justice for the child’s injuries.
Two lawsuits with more on the way have been filed in federal court, the U.S. District Court for the Eastern District of Pennsylvania, alleging serious and potentially fatal birth defects were caused by the mother’s use of Zofran during pregnancy.
Zofran is approved by the FDA for use in treating nausea and vomiting in chemotherapy, radiation, or related to surgery. The drug is administered in oral and IV/injection form. Doctors are allowed to prescribe drugs for unapproved uses, called “prescribing off-label,” if they believe the drug would be of benefit to their patient. The “off-label” use is what is at issue in the lawsuits filed in federal courts.
Studies prompting a growing concern over the use of Zofran during pregnancy have been published in medical journals as recently as February, 2015. One journal, Clinical Pharmacology & Therapeutics, indicate the increasing frequency and serious potential side effects. A previous study reported in the New England Journal of Medicine said there was little risk, but an analysis of the same study by a Canadian physician reported in The Drug Monit found there was double the risk of serious side effects.
This drug has been under scrutiny for some time, due to a 2012 FDA warning that one form of the IV version of the drug could cause serious cardiac defects or an abnormal, potentially fatal heart rhythm. Zofran is no longer used nor marketed in this formula.
Contact Sheller, P.C. by calling 800-883-2299 or using the inquiry form on the bottom of this page. Let us help you protect your child’s current and future health.