High Court Won’t Hear Sheller Dispute with FDA over Risperdal

High Court Won’t Hear Dispute Challenging FDA Over J&J Drug Supreme Court won’t weigh in on whether law firm can sue FDA over drug labeling Circumstances in which third parties can sue over government actions remain unclear By Dana A. Elfin A Philadelphia-based law firm has hit the end of the road in its bid […]
$70M Risperdal Verdict

In the Philly Inquirer July 2, 2016: Philadelphia jury pins $70m verdict on Janssen for its Risperdal drug A Philadelphia jury found Friday that the antipsychotic drug Risperdal caused a Tennessee boy to grow breasts and imposed a $70 million verdict on its manufacturer, Janssen Pharmaceuticals. Lawyers for the boy argued that scientists for the […]
BREAKING: $1.75M Verdict

A $1.75 million verdict in a Sheller firm antipsychotic drug Risperdal case was awarded today in Pennsylvania in the fourth case alleging Johnson & Johnson failed to warn of the risks of gynecomastia, the development of female-like breasts in boys and young men. The jury found the drug was a “substantial factor” in causing a […]
Risperdal News: Sheller Quoted in Toronto Star

Stephen Sheller was quoted in a July 31, 2015 article discussing Risperdal research that was “manipulated by a drug company and understated the risks of a powerful antipsychotic used to treat kids with behavioural problems.” In the news story, pediatrician Denis Daneman says there was no intent on his part to exclude “significant results” from […]
Invokana: FDA Warnings on SGLT2 Diabetes Drugs
Sheller, P.C. attorneys are investigating the newer class of diabetes drugs following FDA warnings. ABOUT THE DRUGS Invokana (Invokamet) is a relatively new SGLT2 inhibitor diabetes drug that is intended to be used in combination with diet and exercise to improve glycemic control and lower blood sugar in adults with type 2 diabetes mellitus. When […]
#1 PA V&S 2013-2014
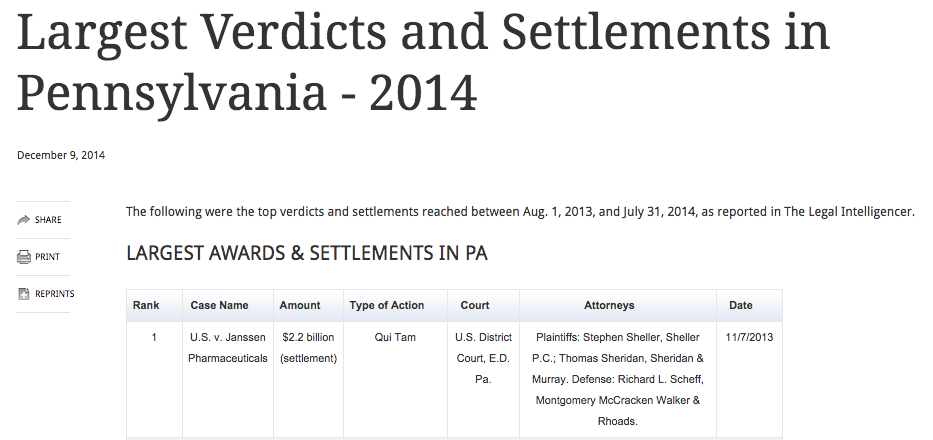
Sheller, P.C. had the largest settlement in Pennsylvania in 2013-2014 as published in American Lawyer Media’s Legal Intelligencer newspaper. The $2.2 billion settlement with Janssen Pharmaceuticals, a division of Johnson & Johnson, was a qui tam case where Sheller, P.C. represented the whistleblower who brought the qui tam charges. With the U.S. Department of Justice, […]
In The Philadelphia Inquirer: Sheller $2.2 Billion J&J Pharma Settlement

J&J to pay $2.2B for Improper Promotion of Risperdal BY DAVID SELL WASHINGTON – Health-care giant Johnson & Johnson agreed Monday to plead guilty to a criminal misdemeanor charge and pay $2.2 billion to settle allegations that it illegally promoted the use of its antipsychotic drugs for unapproved uses, including for children and the elderly. […]
$2.5 Million Verdict
A Philadelphia jury awarded $2.5 million to a twenty year old man whose family claimed he developed female-like breasts (gynecomastia) from taking the antipsychotic drug Risperdal for five years from ages eight to thirteen. Stephen A. Sheller, attorney for the family: “We are encouraged to see this day come, after ten years of our pursuing […]
$2.2B Settlement “Leads the Pack”

The $2.2 Billion Settlement with Johnson & Johnson Leads the Pack by Lizzy McLellan, The Legal Intelligencer The Legal Intelligencer reported that the largest verdict and settlement award in Pennsylvania between August 2013 and August 2014 was handled by Sheller, P.C. with a $2.2 billion settlement over healthcare giant Johnson & Johnson and its subsidiary, […]
$2.2 Billion Settlement

With the $2.2B Risperdal settlement, Sheller, P.C.’s representation of whistleblowers totals over $6.4 billion, with three of the top five pharmaceutical settlements in history 2009: Pfizer $2.3 billion (Geodon) 2009: Eli Lilly & Co. $1.4 billion (Zyprexa) 2013: Johnson & Johnson $2.2 billion (Risperdal, Invega) 2010 : Astra Zeneca $520 million (Seroquel) WASHINGTON, November 4, […]
