Risperdal News: Sheller Quoted in Toronto Star

Stephen Sheller was quoted in a July 31, 2015 article discussing Risperdal research that was “manipulated by a drug company and understated the risks of a powerful antipsychotic used to treat kids with behavioural problems.” In the news story, pediatrician Denis Daneman says there was no intent on his part to exclude “significant results” from […]
Essure Birth Control: Update
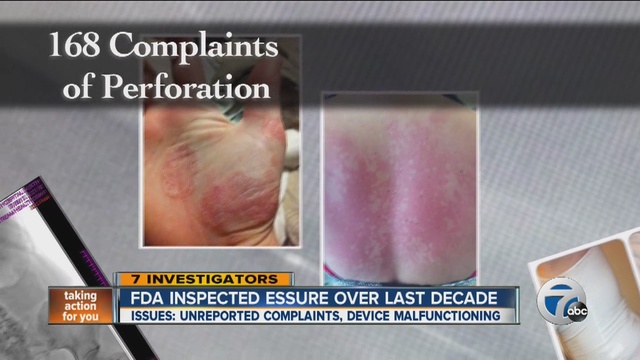
Long term data about the Essure contraceptive device suggest that it may pose risks of serious complications. Essure is a birth control device that consists of two metal coils placed inside the fallopian tubes to block conception. Thousands of women across the country have reported serious side effects associated with the device, including severe and/or chronic pain, […]
Invokana: FDA Warnings on SGLT2 Diabetes Drugs
Sheller, P.C. attorneys are investigating the newer class of diabetes drugs following FDA warnings. ABOUT THE DRUGS Invokana (Invokamet) is a relatively new SGLT2 inhibitor diabetes drug that is intended to be used in combination with diet and exercise to improve glycemic control and lower blood sugar in adults with type 2 diabetes mellitus. When […]
In The New York Times: “Side Effects May Include Lawsuits”

One of the most-read articles in The New York Times regarding pharmaceutical drug product liability and whistleblower lawsuits quotes Stephen Sheller. Side Effects May Include Lawsuits BY DUFF WILSON NEW YORK – FOR decades, antipsychotic drugs were a niche product. Today, they’re the top-selling class of pharmaceuticals in America, generating annual revenue of about $14.6 billion […]





