
Shellers Honored at Mural Arts Philadelphia’s 2021 Wall Ball
Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00

Stephen and Sandy Sheller were honorary attendees at Mural Arts Philadelphia’s 2021 Wall Ball, The Art of Wellbeing. Held virtually June 10, 2021 at 6:00
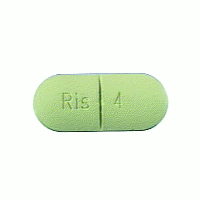
Johnson & Johnson is out of options after the U.S. Supreme Court declined to hear the pharmaceutical company’s appeal of a $70 million Risperdal verdict.

When executive compensation is tied solely to short term profits, and not balanced against company payouts for wrongdoing, there’s an increased incentive for executive fraud.
The FDA has announced that sodium-glucose cotransporter-2 (SGLT2) inhibitors used to treat type 2 diabetes may lead to serious side effects leading to hospitalization. One of the side effects, diabetic ketoacidosis, occurs when the body produces high levels of ketones (blood acids). The metabolic imbalance of insulin already compromised by diabetes is made further dangerous by this excess acid.
Sheller, P.C. attorneys have successfully litigated class action and mass torts against some of the largest corporations in the world, achieving compensation and justice for people nationwide
The laws of our nation are the foundation of our society. The scales of justice are the symbol of the legal system’s mission to balance truth and fairness. While there may be merit for a case, many lawsuits cannot become class action or mass tort lawsuits on their own. But those with a significant number of plaintiffs with similar injuries and common legal issues may be combined and declared a class action or mass tort lawsuit by a judge. Combining numerous separate lawsuits into a class action or mass tort is very efficient- it can ease the load on our judicial system and reduce the amount of time and money spent resolving consumer wrongs.
September 10, 2015: FDA has strengthened the warning for the type 2 diabetes medicine canagliflozin (Invokana, Invokamet) related to the increased risk of bone fractures, and added new information about decreased bone mineral density.
Click here to view the latest Sheller, P.C. blog post on SGLT2 diabetes drugs
First, never discontinue a drug without first consulting your healthcare professional. Stopping suddenly may cause a serious health emergency.
If you have taken Invokana or any of the other SGLT2 inhibitors and experienced a heart attack, stroke, kidney failure or the symptoms of diabetic ketoacidosis (what seems to be a severe case of the flu such as breathing problems, nausea and vomiting, stomach pain, confusion, fatigue and sleepiness), call the national defective drug attorneys at Sheller, PC toll free 800-883-2299, or fill out the inquiry form at the bottom of this page. You may have a case against and eligible for compensation from the manufacturers of SGLT2 drugs for their alleged non-disclosure of the risks you have suffered from the drug.
The label of the drug does not include warnings of some of these life-threatening side effects.
Drugs to treat diabetes are lucrative profit centers for pharmaceutical companies.
In 2013, the top ten diabetes drugs generated $28 billion in sales out of the $35 billion for all diabetes drugs. By 2017, one estimate puts the diabetes drug market at a staggering $55.3 billion worldwide with increasing sales.
Invokana was approved in 2013 for the treatment of Type 2 diabetes. Annual sales of the drug are, according to some reports, in just two years following approval expected to exceed $1.2 billion.
While drugs to treat conditions like diabetes can save lives, there must be high standards for the research, development, testing, and marketing for these drugs.
The Top 10 Best Selling Diabetes Drugs of 2013, Fierce Pharma June 17, 2014 http://www.fiercepharma.com/special-reports/the-top-10-best-selling-diabetes-drugs-2013